As explained on Muscle Radio Episode 34 (https://soundcloud.com/muscleradio) food-specific IgG4 is not a biomarker of food allergy or intolerance in any delayed or immediate responses. The production of food specific IgG4 antibodies is a normal immune system response after exposure to food components. Testing of IgG4 to foods is not reliable nor in anyway diagnostic in the laboratory work-up of food allergy or intolerance and does not provide information that can direct intervention for treating of suspected food related symptoms.
Despite the fact that there is a serious lack of evidence to support IgG4 testing for allergy or intolerance, for me the most deeming evidence for this can be drawn from the interactions of IgG4 with the effectors of the immune cascades that would lead to the overt symptoms and clinical features of a response- be it inflammatory or otherwise.
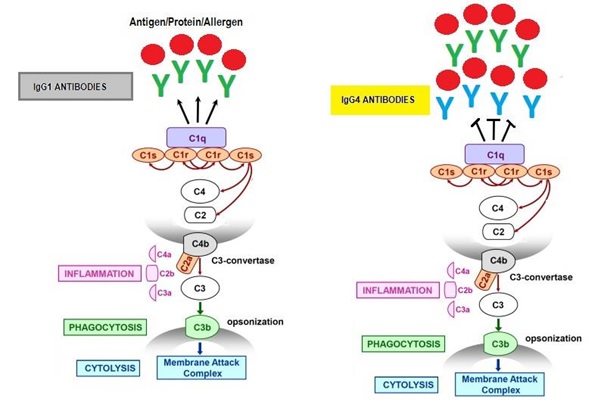
The complement system is a part of the immune system that enhances (or complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, attack a pathogen’s cell membrane and ultimately promote a certain type of inflammation and one type that is typically associated with antibody responses.
Complement component 1q (C1q) is the first component of the C1 complex of the classical pathway of the complement immune system activation. It sort of kicks of the antibody mediated cascade. Each of the IgG antibodies bind to this component and will normally set of the appropriate immune activation depending on the type of antigen or protein bound to the antibody. Of all 4 IgG isoforms IgG4 binding results in the least activation of any type of immune cascade and more often than not will not set of any at all. In fact due to the formation of much smaller immune complexes than the other IgG isomers- it may even be capable of reducing C1q binding of the potent IgG1 antibodies- therefore in this sense may have anti-inflammatory actions.
Fragment crystallizable receptors (FcRs) are found on some of the proteins of the complement system as well as immune cell surfaces. They bind to specific regions on the tails of antibodies- hence are sort of attracted to infected cells, invading pathogens or “allergenic” proteins. The activitation of FcRs stimulates phagocytic or cytotoxic cells to destroy microbes, infected or antigenic cells by antibody-mediated phagocytosis or antibody dependent cell-mediated cytotoxicity. The latter of these would be considered ‘inflammatory’. There are numerous FcRs and as a general rule binding and activating any one of these is lower for IgG4 compared to IgG1. This is not the case for an inhibitory FcR know as the gamma receptor IIb isoform. IgG4 actually has greater affinity for this receptor than does the potent IgG1 and therefore can alter the balance between binding to activating receptors in comparison to inhibitory receptors. What this ultimately means is that this feature of IgG4 may be important in mitigating the pro-inflammatory nature of the other IgGs. Therefore a IgG4 antibody might be necessary to reduce an allergenic response- not promote it.
Caaveiro, J. M., Kiyoshi, M., & Tsumoto, K. (2015). Structural analysis of Fc/Fc?R complexes: a blueprint for antibody design. Immunological reviews, 268(1), 201-221.
Aalberse, R. C., & Schuurman, J. (2002). IgG4 breaking the rules. Immunology, 105(1), 9-19.
Nirula, A., Glaser, S. M., Kalled, S. L., & Taylora, F. R. (2011). What is IgG4? A review of the biology of a unique immunoglobulin subtype. Current opinion in rheumatology, 23(1), 119-124.
Lux, A., Yu, X., Scanlan, C. N., & Nimmerjahn, F. (2013). Impact of immune complex size and glycosylation on IgG binding to human Fc?Rs. The Journal of Immunology, 190(8), 4315-4323.
Bruhns, P., Iannascoli, B., England, P., Mancardi, D. A., Fernandez, N., Jorieux, S., & Daëron, M. (2009). Specificity and affinity of human Fc? receptors and their polymorphic variants for human IgG subclasses. Blood, 113(16), 3716-3725.
Van Der Neut Kolfschoten, M., Schuurman, J., Losen, M., Bleeker, W. K., Martínez-Martínez, P., Vermeulen, E., … & De Baets, M. H. (2007). Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science, 317(5844), 1554-1557.
If you have any questions about a program for you get in touch with us at Schembri PT. You can send us an email to info@schembript.com or fill in this contact us form and we’ll reply as soon as possible.
Thanks for reading,
Schembri PT Team